Every immune system has a story to tell.
The key is knowing how to listen.
Adaptive Biotechnologies was founded on the premise that if you could read how the adaptive immune system detects and treats disease, you could harness these natural abilities to make a difference in the lives of people living with many different diseases.
Pioneering Immune Medicine
The history of medicine is filled with inspiring discoveries and new technologies. But the most powerful tool in the world to keep people healthy isn’t inside a pill or a vaccine. It’s the immune system inside every one of us—working around the clock to identify and treat disease in our bodies.
Now, for the first time, we can look deep inside the human immune response to almost every disease, at scale, to add more perspective and possibility to modern medicine.
The Adaptive A
A Symbol as Unique As Each Person’s Immune System
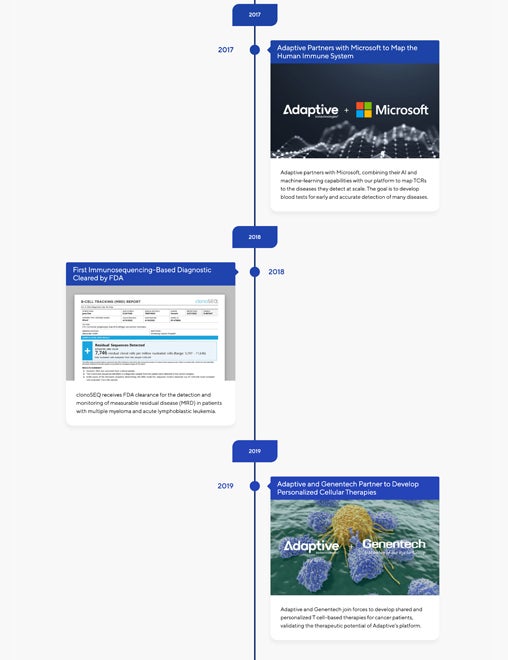
Our Innovation Timeline
Just as our immune systems have evolved, shaped by our environment and biology, so has Adaptive Biotechnologies. Our progress has accelerated our ability to read the immune story within each of us and apply that understanding to improve the diagnosis and treatment of disease. View our timeline to see our history—from our early discoveries to our transformational products and partnerships.
Our mission is to translate the genetic language of the adaptive immune system into clinical products to diagnose and treat disease.
The combination of our unique approach and the near-infinite applications of immune medicine provide opportunities to make a difference in the lives of people living with many different diseases.