Deep detection.
Actionable insights.
Adaptive is the proven leader in measurable residual disease (MRD) testing for lymphoid cancers. FDA-cleared clonoSEQ® is trusted by patients, providers, and biopharma companies for both clinical care and research.
Powered by next-generation sequencing (NGS), clonoSEQ detects and tracks cancer cells that can remain during and after treatment and that may cause disease recurrence.

Baseline sequencing identifies and quantifies patient-specific DNA sequences associated with malignant B or T cells, detecting as few as one cancer cell among 1 million healthy cells (10-6).
Follow-up MRD testing tracks an individual’s disease over time, revealing changes in residual cancer cell levels and providing insight into treatment response.
Unrivaled accuracy and sensitivity provide a clear view of disease dynamics, detecting potential relapses earlier than standard imaging methods like PET/CT, helping clinicians measure treatment effectiveness, and informing therapeutic adjustments when needed.
What is MRD?
The proven standard in MRD for lymphoid malignancies

Comprehensively validated
FDA-cleared in multiple myeloma, chronic lymphocytic leukemia (CLL), and acute lymphoblastic leukemia (ALL); CLIA-validated in diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), and other lymphoid cancers*

Widely reimbursed
- 300 million lives covered by Medicare and every major private insurer
- > 90% of clonoSEQ patients pay $0 dollars out of pocket for testing

Broadly adopted by clinicians
- Ordered by more than 6,100 oncologists in 2025
- Available and actively used at 1,800 leading academic and community centers, including all 33 National Comprehensive Cancer Network (NCCN) cancer centers
- More than 82,000 clonoSEQ test results delivered in 2025 to inform treatment decisions

Recommended by practice-defining guidelines
NGS-based MRD testing is incorporated in NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) ALL, CLL, DLBCL, MCL and multiple myeloma

Extensively studied
More than 250 peer-reviewed publications in ALL, CLL, DLBCL, MCL and multiple myeloma
Trusted by biopharma
Used in more than 175 biopharma-sponsored trials, including 15+ studies in which it is used as a primary endpoint to support drug development
Clarity to inform individualized care
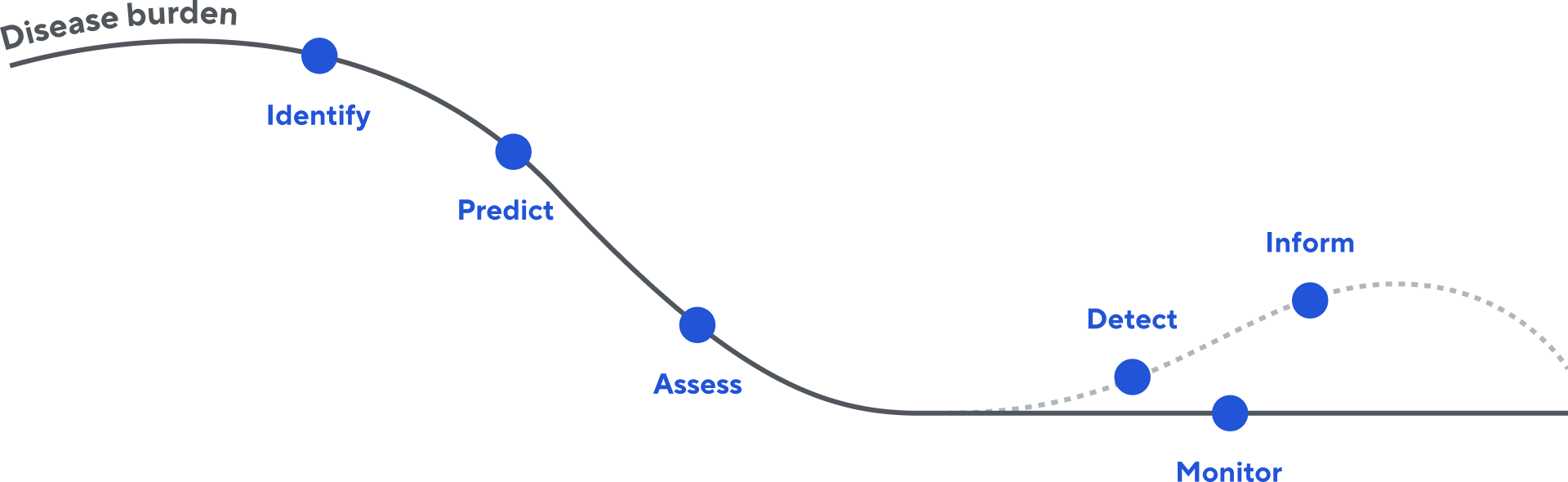
Disease burden assessment is essential to clinical decision-making throughout the treatment continuum. clonoSEQ is shaping modern lymphoid cancer care—enabling clinicians to individualize therapy, lessen treatment burden, and help patients reach their treatment goals.
Integrate highly-sensitive MRD testing at key care timepoints to monitor and detect potential relapse early
Experts in MRD-informed clinical trials

Adaptive partners with more than 50 biopharma companies to accelerate the development of the next generation of lymphoid cancer therapies.

MRD is increasingly recognized as a surrogate endpoint helping accelerate the evaluation of new therapies.
Regulatory agencies including the FDA’s Oncologic Drugs Advisory Committee (ODAC) and the EMA’s Committee for Medicinal Products for Human Use (CHMP) have acknowledged the value of MRD in assessing treatment response and clinical benefit.1,2

By providing an earlier measure of therapeutic efficacy and a clearer view of response depth and duration, clonoSEQ is reshaping how novel treatments are evaluated.
clonoSEQ is reshaping how novel therapies are evaluated

Streamlined clinical workflows
Adaptive electronic medical record (EMR) integration streamlines workflows so healthcare providers can focus on delivering better care. With integration available for most major platforms, Adaptive makes it easier to place orders, reduce manual entry errors, and access results in the patient’s chart.
Knowledge that empowers blood cancer patients
After a decade of maintenance chemotherapy for multiple myeloma, Karen was told she’d need treatment indefinitely—despite side effects impacting her quality of life. After achieving MRD negativity based on clonoSEQ testing, she and her physician altered her maintenance plan to eliminate chemotherapy, giving her back time, energy, and hope.
Karen later said of her experience, “You gave my life back to me.” clonoSEQ doesn’t just provide data—it enables choices.
*clonoSEQ® is available as an FDA-cleared in vitro diagnostic (IVD) test service provided by Adaptive Biotechnologies to detect measurable residual disease (MRD) in bone marrow from patients with multiple myeloma or B-cell acute lymphoblastic leukemia (B-ALL) and blood or bone marrow from patients with chronic lymphocytic leukemia (CLL). clonoSEQ is also available for use in other lymphoid cancers and specimen types as a CLIA-validated laboratory developed test (LDT). To review the FDA-cleared uses of clonoSEQ, visit clonoSEQ.com/technical-summary.
- U.S. Food and Drug Administration. Oncologic Drugs Advisory Committee Meeting: Use of Minimal Residual Disease (MRD) as an Endpoint in Multiple Myeloma Clinical Trials. April 12, 2024. Accessed November 12, 2025. https://www.fda.gov/media/180109/download.
- International Myeloma Foundation. “The International Myeloma Foundation Proudly Announces the Completion of EMA-CHMP’s Qualification Advice to i2TEAMM Novel Biomarker Procedure Application on the Use of MRDnegCR as an Intermediate Early Endpoint for Conditional Market Approval in Myeloma Clinical Trials.” News release, July 1, 2025. Accessed November 12, 2025. https://www.myeloma.org/news-events/multiple-myeloma-news/imf-proudly-announces-ema-chmp-positive-qualification-advice-i2teamm-novel-biomarker-procedure.