Identifying and Advancing Therapeutic TCRs
Our T-cell receptor (TCR) discovery approach allows us to identify naturally-occurring, potent TCRs for clinical development and commercialization with partners or on our own. To date, we have identified and characterized more than 8,000 unique antigen-specific TCRs against 600 clinically relevant targets. This represents a massive library of therapeutic grade TCRs.
Differentiators of Adaptive’s Approach

Versatility
- Use blood samples
- Target any antigen (the entire proteome)
- Class I and II HLAs

Safety
- Naturally-occurring, fully human TCRs
- TCRs that have undergone thymic selection

High-throughput, high sensitivity
- Test 1000s of antigens in a single screen
- Identify ultra-low frequency TCRs
- Accurate pairing of 100s of 1000s of TCR alpha/beta chains

Selection of best clinical candidates
- Query entire TCR repertoire
- Evaluate well-characterized, naturally-optimized, cytotoxic TCRs
- Flexibility for multi-targeting by using combinations of potent TCRs
Cancer Cellular Therapy
Collaboration with Genentech
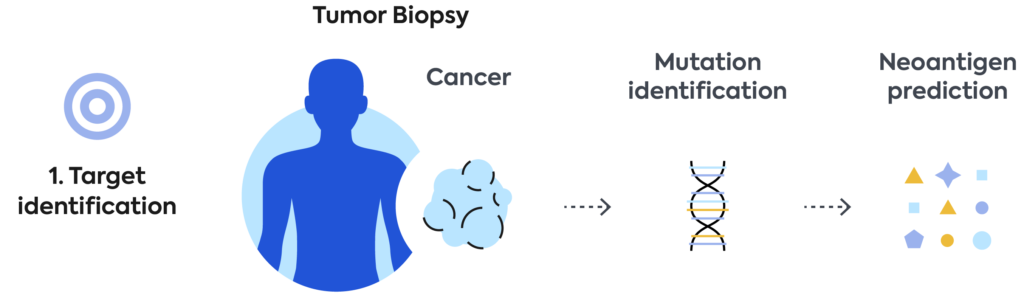
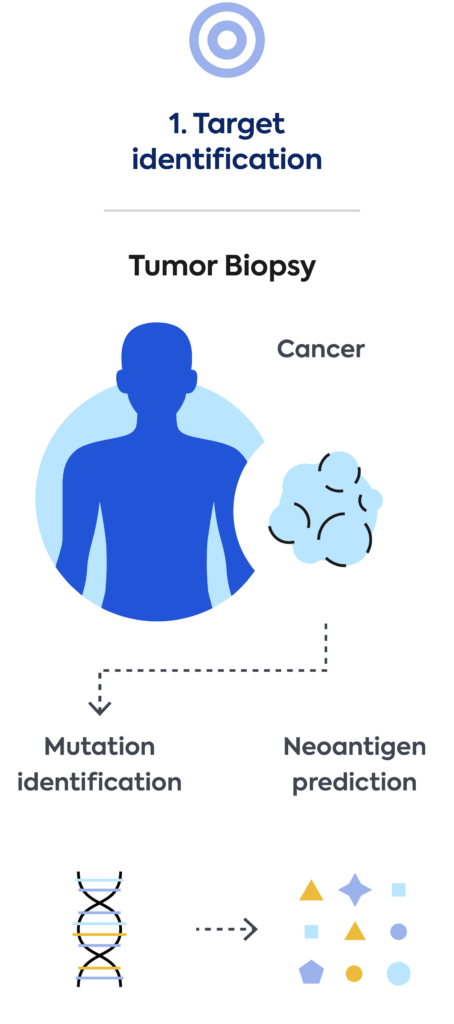
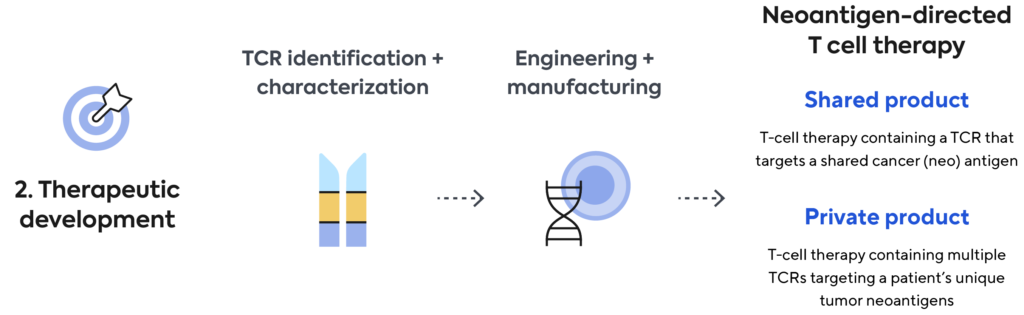
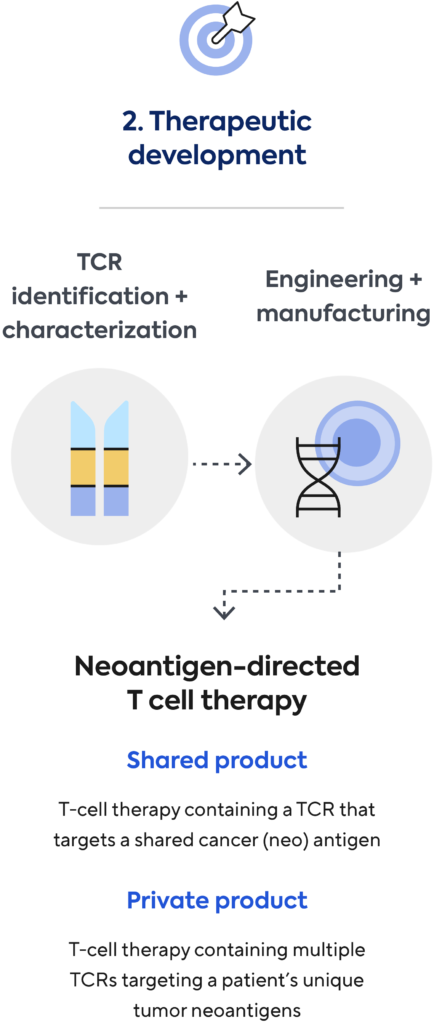
We’re developing, manufacturing, and commercializing novel neoantigen directed T-cell therapies for the treatment of a broad range of cancers. We aim to build a transformational new treatment paradigm to tailor cellular therapy for each patient’s individual cancer.
Shared Product
We use our immune medicine platform to screen, identify, and characterize TCRs to shared cancer antigens commonly found in many cancer patients. For our shared product approach, we use blood from healthy donors. TCRs discovered by Adaptive are then engineered into T cells and manufactured as an off-the shelf cell therapy product.
Private Product
Using a patient’s own blood, Adaptive screens and identifies in real-time the best neoantigen-specific TCRs targeting that patient’s unique tumor mutations. Genentech will use these patient-specific TCRs to engineer and manufacture a personalized cellular medicine and deliver it to each patient.